About MSG Safety & Benefits
What is MSG? MSG stands for 'Monosodium Glutamate'. It is simply a combination of 12% sodium, 78% glutamate and 10% bound water. Glutamate is an amino acid that provides Umami taste. It is found abundantly in protein rich foods such as meat, fish, poultry, milk (including human breast milk) etc.
Glutamate can be produced naturally in our bodies, such as in the muscle, brain and other organ tissue. It plays an important role in metabolism.
Let's discover more on the interesting facrs about MSG as below:
FAQ
-
What is Monosodium Glutamate?
MSG is a flavour enhancer/ Umami seasoning, which has been widely used for nearly a century to bring out the best flavour in foods. MSG is actually made of the most abundant amino acid known as glutamic acid or glutamate for short, sodium and water. Glutamate is found naturally in protein-containing foods, such as meat, vegetables, poultry, and milk. The human body also produces glutamate naturally in large amounts. The muscles, brain, and other body organs contain about four pounds of glutamate. Breast milk is rich in glutamate – 6 times higher compared to cow’s milk, for example.
-
How is MSG made?
MSG is produced through a natural process called fermentation – a process similar to the making of vinegar and yogurt. MSG production begins with the fermentation of tapioca or sugar cane, followed by neutralisation and crystallisation process. The finished product is a pure, white crystal, which easily dissolves and blends well in many foods.
-
Is MSG safe?
Yes. MSG has been used for more than a hundred years and is one of the most studied of all food ingredients. It extensive database of hundreds of peer-reviewed studies has undergone intensive scrutiny and MSG’s safety has been confirmed time after time in reviews of this data.
For more than four decades, the Food and Drug Administration (FDA) has repeatedly confirmed MSG’s safety.Other scientific and regulatory bodies around the world, including the American Medical Association (AMA), the European Communities Scientific Committee for Food (SCF), and the Joint FAO/WHO Expert Committee on Food Additives (JECFA), have also reviewed the research on MSG and have affirmed its safety. MSG is so safe that the FDA has placed it on the list of substances known as “Generally Recognized As Safe” (GRAS), right along with sugar, baking powder, salt, and pepper. Likewise, the World Health Organization (WHO) has chosen not to set a limit on the Acceptable Daily Intake (ADI) of MSG, classifying it as “not specified”.
-
What is the consumption limit of MSG per day?
There is no limitation for the use of MSG as a food additive because scientific and regulatory bodies such as the Joint FAO/WHO Expert Committee on Food Additives (JECFA)* placed MSG in the safest category “Acceptable Daily Intake (ADI) not specified” based on the extensive scientific data.
Furthermore, MSG is a self-limiting substance. Once the proper amount is used, additional use contributes little, or not at all, to food flavor. In fact, adding too much MSG can result in a decline in palatability and is economically wasteful.
-
Is MSG safe for children and pregnant women?
Yes, scientific and regulatory authorities worldwide, including the Food and Drug Administration (FDA), the American Medical Association (AMA), the European Commission Scientific Committee for Food, and the Joint Expert Committee on Food Additives (JECFA), have reviewed the research on MSG and have repeatedly affirmed its safety for the general population, including children and pregnant women.
-
Are people sensitive to MSG?
No. MSG is not an allergen, according to the American College of Allergy, Asthma and Immunology. The U.S. Food and Drug Administration has found no evidence to suggest any long-term, serious health consequences from consuming MSG. It is possible that some people might be sensitive to MSG, just as to many other foods and food ingredients. There are some reports that mild, temporary reactions to MSG may occur in a small portion of the population, based on tests with a large dose of MSG in the absence of food.
-
Does MSG cause baldness or hair loss?
There is no scientific support for this claim. Baldness or hair fall may be caused by several factors, namely: Genetic; stress and social pressures; medication; other hair related factors, for example, scalp and hair infection from fungi, and more.
-
Is MSG related to the Chinese Restaurant Syndrome (CRS)?
Chinese restaurant syndrome describes transient discomfort some people may feel after ingesting certain foods and beverages. In 1987, JECFA concluded that “Properly conducted double-blind studies among individuals who claimed to suffer the syndrome did not confirm monosodium glutamate as the causal agent”. (Joint FAO/WHO Expert Committee on Food Additives. L-glutamic acid and its ammonium, calcium monosodium and potassium salts. In Toxicological Evaluation of Certain Food Additives and Contaminants. WHO Food Additives Series No. 22, New York: Cambridge University Press, pp. 97-161, 1988.) This conclusion was affirmed by a comprehensive review and strictly controlled study at the University of Western Sydney in 1993. (Tarasoff L, Kelly M. F. Monosodium L-glutamate: a double blind study and review. Food Chemical Toxicology, 31: 1019 •1035, 1993.
-
Does MSG cause thirst?
There is no scientific support for this claim; Thirst happens naturally when body fluid control is imbalance. Imbalance can caused by severe loss of blood; sweat during a very strenuous exercise; high sodium intake. Looking at the cause of high sodium intake, of course, MSG contains sodium. However, MSG contains only 12% of sodium, whereas table salt contains approximately 40%, and the amount of MSG used for cooking can be far less than that for salt, the thirst-response to MSG if any should far less than that to salt.
-
Does MSG cause migraine headaches?
No. Migraine headaches are severe, often debilitating headaches. While there are many theories about what causes migraines, such as heredity, neurological brain disorder, and blood vessel disorders, there are no double-blind, placebo controlled studies linking MSG to migraines.
Some studies have implicated MSG as a trigger for headaches. One 1991 study sought to establish this connection through MSG elimination diets and self-reported symptoms, rather than the double-blind challenge technique considered more appropriate in clinical research of this nature.There are many known “triggers” for headaches, including diet and stress. A wide array of foods has been implicated as headache triggers. However, a 1990 critical review of the literature on food-triggered headaches concluded that the relationship is controversial. The review further states that there is no evidence to support an association between MSG and migraine headaches.
The exact cause of migraine is not fully understood. Some researchers believe that migraine is rooted in a neurological disorder in the brain, others believe that it is a blood vessel disorder. Hereby also plays role in migraine headaches.
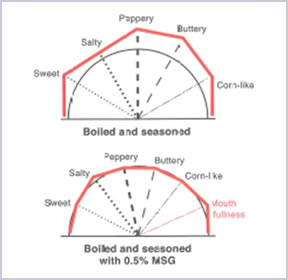
1. Enhance The Flavour Of Food
(Cairncross and Sjostrom, 1950; J.E.Caul, 1957)
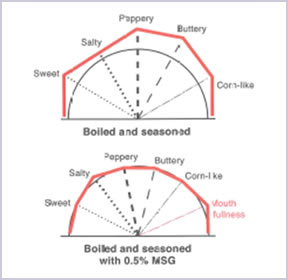
With the usage of AJI-NO-MOTO®, it enhances the flavour of the foods and harmonises all the taste on the meal to give a balance and rich flavour that whet the appetite for the next bite.
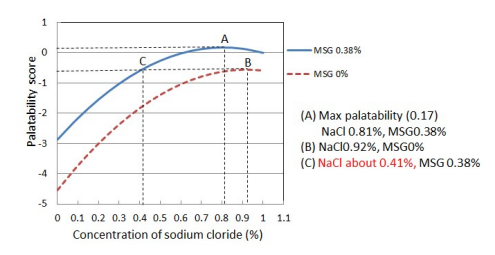
2. Reduce Salt/Sodium Intake In Daily Diet
(S. Yamaguchi and C. Takahashi, 1984)
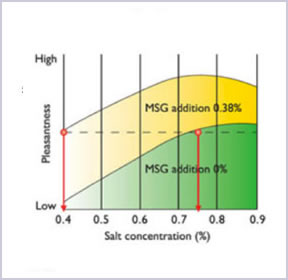
AJI-NO-MOTO® contains only 12% of sodium while table salt contains 39%. By comparing the amount of sodium, AJI-NO-MOTO® is 2/3 less sodium than table salt. Therefore, by replacing part of table salt with a small amount of AJI-NO-MOTO®, overall sodium intake can be reduced up to 40% while maintaining the same deliciousness.

3. Promote Nutrient Intake Of The Elderly
(F. Bellisle, 1991)
As human grow older, the taste buds are getting less sensitive and this leads to lack of appetite which may result mal-nutrients for the elderly. With the usage of AJI-NO-MOTO®, we are able to promote a healthier life of the elderly by improving their appetite, thus increasing their food intake and improve their nutrient absorption.
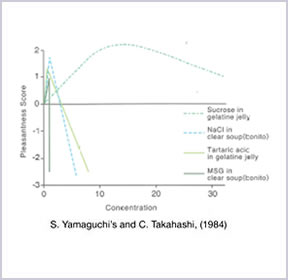
4. Avoid Wastage
(S. Yamaguchi and C. Takahashi, 1984)
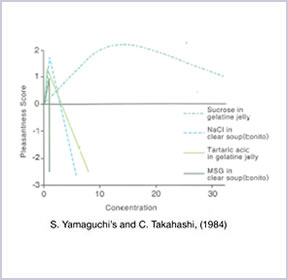
AJI-NO-MOTO® is a ‘self-limiting’ seasoning. An optimum amount of AJI-NO-MOTO® is enough to provide rich Umami taste. Therefore, additional usage will result in a decline in palatability and economically caused wasted.
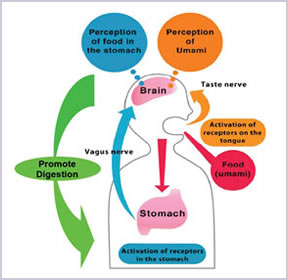
5. Promote Protein Digestion
(Uneyama H, Niijima A and SanGabriel A, et al, 2006)
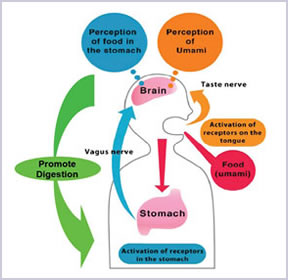
Scientists discovered that using AJI-NO-MOTO® could help to improve the quality of the life as umami receptors was found in the stomach which play an important role in ingestion, digestion/absorption and metabolism of protein that are essential for life.
Since 1909, AJI-NO-MOTO® seasoning has been safely used throughout the world and it is also one of the most extensively researched food ingredients with more than hundreds of Scientifics studies affirmed the safety of MSG consumption. Below are the lists of organisations that have declared the safety of MSG consumption:
Joint Expert Committee on Food Additives (JECFA)
A scientific advisory body to the World Health Organization (WHO) and Food and Agriculture Organization of the (FAO) United Nations. For more info »
In 1987, JECFA has declared the “Acceptable Daily Intake” (ADI) for MSG to be “not specified”
U.S Food Drug Administration (US FDA)
An agency of the United States Department of Health and Human Services, one of the United States federal executive departments. For more info »
FDA had reaffirmed the safety of MSG in 1995 after Federation of American Societies for Experimental Biology (FASEB) reviewed and found no evidence linking MSG to any serious long term health effects which was commissioned by FDA.
European Commission’s Scientific Committee for Food (SCF)
Established on 1974 and consist of highly qualified committee members in fields associated with medicine, nutrition, toxicology, biology, chemistry and others. For more info »
European Commission’s Scientific Committee for Food (SCF) has confirmed the ADI for L-Glutamic acid or monosodium glutamate is not specified” based on the data provided and in view of the large normal dietary intake of glutamate
Food Standard Australia New Zealand (FSANZ)
An independent statutory agency established by the Food Standards Australia New Zealand Act 1991. FSANZ is part of the Australian Government’s Health and Ageing portfolio. For more info »
In 2002, Food Standards Australia New Zealand conducted a review of the safety of MSG. The Chief Scientist concluded that several reviews of the scientific evidence have confirmed that MSG is safe for general public at the levels of use typically found in food.
Read more about MSG in Food here »
Download info about Monosodium Glutamate here »
American Academy of Allergy, Asthma & Immunology (AAAAI)
A professional organisation with more than 6,700 members in the United States, Canada and 72 other countries with a special interest in the research and treatment of allergic and immunologic diseases. For more info »
There were no reactors in MSG found to be associated with the provocation of Asthma attack
Malaysia Food Acts 1983 and Food Regulations 1985
The Food Act 1983 and the Food Regulations 1985 are the Malaysian food legislations that form the backbone of the food safety programme which is essential in establishing an effective food safety system.
Monosodium Glutamate is classified under the category of “Permitted Flavour Enhancer”
Scientific Reports from International Scientific Associations
- Attempts to Establish the Safety Margin for Neurotoxicity of Monosodium Glutamate
(L. Airoldi, A. Bizzi, M. Salmona, and S. Garattini) - Biochemical Aspects of the Neurotransmitter Function of Glutamate
(R. P. Shank and M. H. Aprison) - Biochemical Studies on Glutamate Taste Receptors: The Synergistic Taste Effect of L-Glutamate and 5’-Ribonucleotides
(Robert H. Cagan, Kunio Torii, and Morley R. Kare) - Biochemistry of Glutamate: Glutamine and Glutathione
(Alton Meister) - Central Nervous System Receptors for Glutamic Acid
(Graham A. R. Johnston) - Comparative Metabolism of Glutamate in the Mouse, Monkey and Man
(L. D. Stegink, W. Ann Reynolds, L. J Filer, Jr. G. L. Baker, T. T. Daabees, and Roy M. Pitkin) - Effects of Glutamate Administration on Pituitary Function
(Alan F. Sved and John D. Fernstrom) - Excitotoxic Amino Acids: Research Applications and Safety Implications
(John W. Olney) - Factors Affecting Plasma Glutamate Levels in Normal Adult Subjects
(Lewis D. Stegink, L. J. Filer, Jr. ,G. L. Baker, S. M. Mueller, and M. Y-C, Wu-Rideout) - Factors in the Regulation of Glutamate Metabolism
(Hamish N. Munro) - Factors Influencing Dicarboxylic Amino Acid Content of Human Milk
(G. L. Baker, L. J. Filer, and L. D. Stegink) - Food-Symptomatology Questionnaires: Risks of Demand-Bias Questions and Population-Biased Surveys
(George R. Kerr, Marion Wu-Lee, Mohamed El-Lozy, Robert McGandy, and Frederick J. Stare) - Free & Bound Glutamate in Natural Products
(T. Giacometti)
- Glutamate in the Striatum
(E. G. McGeer, P. L. McGeer, and T. Hattori) - Glutamate Metabolism and Placental Transfer in Pregnancy
(Roy M. Pitkin, W. Ann Reynolds, L. D. Stegink, and L. J. Filer, Jr.) - Glutamate Toxicity in Laboratory Animals
(R. Heywood and A. N. Worden) - Glutamic Acid as a Transmitter Precursor and as a Transmitter
(E. Costa, A. Guidotti, F. Moroni, and E. Peralta) - In Utero and Dietary Administration of Monosodium L-Glutamate to Mice: Reproductive Performance and Development in a Multigeneration Study
(K. Anantharaman) - Metabolism of Free Glutamate in Clinical Products Fed Infants
(L. J. Filer, Kr., G. L. Baker, and L. D. Stegink) - Morphology of the Fetal Monkey Hypothalamus After In Utero Exposure to Monosodium Glutamate
(W. Ann Reynolds, Naomi Lemkey-Johnston, and Lewis D. Stegink) - Placebo-Controlled Studies of Human Reaction to Oral Monosodium L-Glutamate
(Richard A. Kenney) - Problems in the Evaluation of Glutamate as a Central Nervous System Transmitter
(D. R. Curtis) - Psychometric Studies on the Taste of Monosodium Glutamate
(Shizuko Yamaguchi and Akimitsu Kimizuka) - Regulation of Amino Acid Availability to Brain: Selective Control Mechanisms for Glutamate
(William M. Pardridge) - Self-selection of Food and Water Flavored with Monosodium Glutamate
(Michael Naim) - Toxicological Studies of Monosodium L-Glutamate in Rodents: Relationship Between Routes of Administration and Neurotoxicity
(Yutaka Takasaki, Yoshimasa Matsuzawa, Seinosuke Iwata, Yuichi O’hara, Shinobu Yonetani, Masamichi Ichimura)